Tendon Healing Mechanobiology
– Lennard Funk - 2007
Introduction
Tendons are mechanically responsible for transmitting muscle forces to bone and in doing so permit locomotion and enhance joint stability.
Tendons consist of collagens, proteoglycans, glycoproteins, water and cells. Tendons are rich in collagens with the most abundant tendon component being type I collagen, constituting about 60% of the tendon and about 95% of the total collagen. The remaining 5% consist of type III and V collagens. In normal tendons, type III collagen is mainly located in the endotenon and epitenon. However, it is also found in aging tendons and at the insertion sites of highly stressed tendons such as the supraspinatus. Type III collagen forms smaller less well organised fibrils and type I collagen, which may result in decreased mechanical strength.
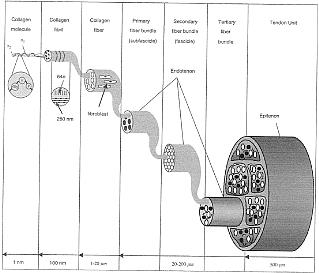
Tendon Structure:
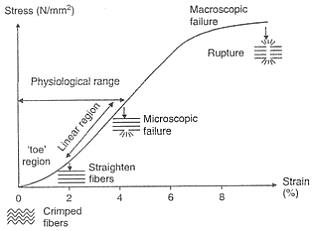
Stress-strain curve for tendon:
Tendons are living tissue and respond to mechanical forces by changing the metabolism as well as their structural and mechanical properties. For example, tendons exhibit increase cross sectional area and tensile strength, with increased tendon fibroblast production of collagen type I in response to appropriate physical training. However, inappropriate physical training leads to tendon overuse injuries or tendonopathy and excessive repetitive stretching of tendon fibroblast increases the production of inflammatory mediators such as prostaglandin E2 and leukotriene B4. The ability of connective tissues like tendons to alter their structure in response to mechanical loading is referred to as tissue mechanical adaptation.
Forces generating muscles are transmitted to bone through tendons, which makes joint and limb movement possible. To do this effectively, tendons must be large forces, but the level of muscle contraction and the tendons relative size influence mechanical forces on the tendon. In general, the greater the cross sectional area of the muscle, the higher force it produces and the larger stress a tendon undergoes. Different activities induce different levels of forces even on the same tendon and varying the rate and frequency of mechanical loading results in different levels of tendon forces.
Because of their elasticity, tendons are more deformable at low strain rates. Therefore, the tendons absorb more energy but are less effective in transferring loads. At high strain rates, tendons become less deformable with a high degree of stiffness and are more effective in moving large loads.
Training induces biomechanical changes in tendons. Strenuous endurance training increases collagen turnover but decreases collegan maturation in tendons. Exercise stimulates tenocytes to increase the expression of Insulin-like Growth Factor 1
(IGF-1). IGF-1 is a potent stimulus of collegan synthesis and cell proliferation. As such, the IGF-1 may serve as a protein marker for tendon remodelling activities. This results in a net synthesis of type I collegan production. There have been few studies to determine the affects of tendon disuse and immobilisation; however, it is known that the affect of disuse and immobilisation on tendons is much slower and less dramatic than on skeletal muscles because they have a much slower metabolism and vascularity. In general however, immobilisation decreases the total weight of the tendon, stiffness and tensile strength. Studies have shown that immobilisation caused the formation of irregular and uneven collagen fibres, dilated veins and capillaries. Stress deprivation due to immobilisation has been thought to be responsible for the degenerative changes in tendons.
Tendon healing process
Tendon healing can be largely divided into 3 overlapping phases, inflammatory repairing and remodelling phases:
The initial inflammatory phase, which lasts about 24 hours, erythrocytes, platelets and inflammatory cells (eg: neutrophils, monocytes and macrophages) migrate to the wound site and clean the site of necrotic materials by phagocytosis. In the meantime, these cells release phaso active and chemo tactic factors which recruit tendon fibroblast to begin collegan synthesis and deposition.
A few days after the injury, the repairing phase begins. In this phase, which lasts a few weeks, tendon fibroblast synthesise abundant collegan and other extra cellular matrix components such as proteoglycans and deposit them at the wound site.
After about 6 weeks, the remodelling phase starts. This phase is characterised by decreased cellularity and decreased collagen and glycosaminoglycan synthesis. During this period, the repair tissue changes to fibrous tissue, this again changes to scar like tendon tissue after 10 weeks. During the later remodelling phase covalent bonding between the collagen fibres increases resulting in repaired tissue with highest stiffness and tense our strength. Also, both the metabolism of tenocytes and tendon vascularity decline.
During tissue healing growth factors play an important role in this process.
1: Platelet Derived Growth Factor (PDGF) is produced shortly after tendon injury and stimulates the production of other growth factors.
2: TGF-beta is active during the inflammatory and repair phases of tendon healing. TGF-beta plays a major role in the repair of injured tendons. TGF-beta 1 aids an extra cellular matrix deposition; however, it’s over expression results in tissue fibrosis. TGF-beta 2 functions similarly to TGF-beta 1. However, TGF-beta 3 has been shown to improve tissue scarring. Peak levels of TGF-beta receptory expression occur at day 14 post injury and decrease until day 56 post injury.
3. Vascular Endothelial Growth Factor (VEGF) stimulates endothelial cell proliferation, enhances angiogenesis and increases capillary permeability. VEGF RNA expression is detected at the repair site 7 days post injury with peak levels at 10 days post injury.
4. Nitric Oxide Synthase (NOS) isoforms are expressed with differential expression patterns during the 3 phases of tendon healing.
It should be noted that, except for degenerative tendons (tendonosis), injured tendons tend to heal. However, the healing tendon does not reach the biomechanical properties of the tendon prior to surgery.


